RHESUS - SYSTEM
The Rhesus blood group system is by far the largest. It includes 55 different antigens=blood groups that could trigger the formation of antibodies. The most important antigen in this system is D. It is still the leading cause of haemolyticus neonatorum. Therefore, a transfusion of Rhesus-positive blood to girls and women of childbearing age who are themselves Rhesus-negative should only occur in vital care. Furthermore, it must be established beforehand that such immunisation has not already taken place. A transfusion against an anti-D can have similar consequences as an incompatible transfusion in the ABO system.
Otherwise, men and non-childbearing women, as well as male children who are themselves rhesus-negative, may be transfused with rhesus-positive blood. But this also applies ONLY in the case that
- there is a real shortage of O (rhesus negative) red cell concentrates
- and/or a high consumption is to be expected
There is no antithetic antigen for the D - rhesus negative therefore means that the large D is missing.
The clinically relevant antigens in the Rhesus system include C, the antithetic c, and E and the antithetic e. Antithetical antigens to each other are different alleles of the same gene. The difference is almost always a nucleotide exchange in the gene sequence. In addition, another antigen, called Cw, is found on some C-positive cells. These cells are usually, but not always, rhesus positive.
In the rhesus system, antigen-identical or antigen-compatible transfusions should be performed for all main antigens, but this is not done - probably for financial and logistical reasons. However, suppose a patient has already developed an alloantibody. In that case, the entire rhesus system (all five antigens, e.g., CCDee) is considered from then on - this was regulated in the guidelines.
Here is an example of the clinical significance of irregular antibodies to red cell antigens.
(and ALWAYS assuming the patient does NOT have irregular antibodies yet).
A positive - it is one of the most common blood groups, and the patient can be managed without any problems.
But what can be hidden behind the term A+?
Since we want to stay with the Rhesus, let the blood group A be blood group A (there are also variants), but with the Rhesus phenotype, there are many variants that are nevertheless all Rhesus positive: ccDee (quite rare), CCDee (frequent) CcDee (very frequent), CCDEE (very rare), CcDEe (relatively frequent), ccDEE (rare) - if you want, you can try all the possibilities. Here we can already see that there can be many discrepancies between the donor and the recipient, and for every antigen of the donor erythrocytes, the recipient, if he does not have the antigen himself, can form antibodies against it. And then a simple A pos patient can also become a "treatment problem".
So we take the same patient with BG A positive. Only this time, he is already pre-transfused and has developed the antibody anti-e. At this point, at the latest, the patient's rhesus phenotype is examined.
His rhesus phenotype: ccDEE - only 2% of rhesus-positive have this phenotype.
Having developed an antibody before, the likelihood of antibody formation has increased - statistically and actually from self-observation - no textbook knowledge.
So the first thing they will do is try to transfuse him with the same phenotype - ccDEE. But unfortunately, there are only two red cell concentrates with this rhesus phenotype in the blood depot; of these, one EC already has the BG O. The surgeon needs at least 4 ECs. If you are "lucky", you will find a reserve with CcDEE or ccddEE - frequencies 0.06% and < 0.01%, respectively.
Now the patient has been brought through the operation. He is recovering well. Only the haemoglobin does not want to normalise. It remains constant at 7.4 g/dl and 22% haematocrit. So the doctor on the ward orders two more red cell concentrates for him.
However, the antibody screening test shows that the reaction pattern cannot match the anti-e alone. So antibody differentiation is carried out again, and this time, the patient has indeed developed an anti-C (he was given the one rare EC with CcDEE during the operation).
Having these two antibodies only in the Rhesus system, the further blood supply is no longer uncomplicated. The patient needs blood of group A or O with the Rhesus phenotype: ccDEE. And you hope that he won't develop any additional antibodies against one of the 350 other blood groups... So far, we stayed within the Rhesus system.
By the way, this is a situation where the administration of "0 negative" - from the so-called universal donor - can send the patient into severe haemolysis. Rhesus negative usually means ccddee, and our patient has anti-e antibodies. The antibody will not cause immediate intravascular haemolysis most of the time. Nevertheless, delayed extravascular (spleen, liver) haemolysis can also cause severe renal dysfunction and bilirubin rise.
For this reason, transfusion of uncrossmatched blood should be be avoided as best as possible. Each blood unit administered in this way is " crossmatched " later - the compatibility test with the patient's serum, which must be taken BEFORE the first transfusion was given, is carried out afterwards. If this crossmatch is positive, the patient must be monitored for haemolysis and treated accordingly if necessary.
Of course, the question now arises as to why all antigens are not taken into account from the beginning - or at least the "most important" ones. At large blood depots (Innsbruck, Linz, Vienna), this is precisely how it is done, at least for children and women of childbearing age. But hospitals with a small depot will not always have such blood units available.
In addition, only about 1-2% of all transfused patients develop antibodies. And most pregnant women will never hear of "antibodies" either.
If you work in such an institution, you automatically get the feeling: all patients develop antibodies. And every pregnant woman has to fear for her child for the entire pregnancy.
This is simply because we - transfusion specialists - only get to see such patients - the others, without antibodies, are transfused and have healthy babies, only we don't see them.
DISTRIBUTION AMONG THE POPULATION
In Europe, 85% of people are Rh positive and 15% Rh negative.
In Asia and Africa, rhesus-negative people are practically non-existent! The people there are almost 100% rhesus positive!
NOMENCLATURE AND INHERITANCE
The notation of the rhesus phenotype used in Europe is based on Fisher/Race.
It writes out all 5 or 6 possible antigens - with one exception in RhD, where, as we have learned, there is only one antigen - if it is missing, it is simply not there and "imaginary" antigens - dd - are written down.
In rhesus-positive individuals, only one antigen is reported - D, because it does not matter phenotypically whether someone is homozygous or heterozygous for D.
So different phenotypes (rhesus formula) can look like this:
- ccddee - clearly RhD negative - the two small dd do not exist.
- Ccddee - RhD negative
- CcD.ee - is the most common rhesus constellation of all - Rh positive
According to Wiener's American spelling, these phenotypes would look like this:
- rr
- r'r
- R'r
This notation considers the haplotypes, i.e. the antigens inherited from one parent. These are inherited as a unit: the RHCE and the RHD gene are linked. The gene product of RHCE can be ce, cE, CE or Ce. The product of the RHD gene is then added - D if D is expressed and d if it is absent.
The haplotype is then represented as the sequence of the three letters (in alphabetical order for simplicity):
cde, CDe, cDe, etc.
The European notation ccddee or CCDee throws both haplotypes together - in this way, it is unclear which antigen belongs to which haplotype. But also, the American notation is often only a statistical probability of what the haplotype could look like.
Example:
CcDee would be called R'r.
The following is assumed: 1st haplotype: CDe, 2nd haplotype: cde.
These are the most frequent two that result in this combination.
But other combinations result in CcDee:
- cDe and Cde - R0r'.
- Or CDe and cDe - R'R0.
But R'r is the most likely combination.
The phenotypic result is the same in all cases. The person is Rhesus D positive and also expresses C, c and e on their red cells. What the phenotypes look like can only be determined with molecular genotyping methods.
So what about inheritance? A nice catch question to make residents sweat: Can rhesus-positive parents have a rhesus-negative child?
For someone to test rhesus positive in serological blood grouping, the RHD gene only has to be expressed in one of the two haplotypes. If we now take the example above for both parents:
Mum and Dad: CcDee - most likely combination of haplotypes: CDe and cde. If the child gets the cde haplotype from both parents, it is rhesus negative.
So yes, it is possible! And vice versa? Can rhesus-negative parents have a rhesus-positive child?
No, they can't. Because where would the child get the RhD from?
All clear?
CLINICAL RELEVANCE
Immunisation to rhesus D antigen in a rhesus-negative woman is the most common cause of haemolyticus fetalis or neonatorum.
Since the introduction of rhesus prophylaxis, the risk of immunisation has been reduced to a minimum. It affects women who refuse vaccination - fortunately, only a few, vaccination failures - also rarely. Unfortunately, the largest group are women who give birth to their first child in a country where medical care, if it exists, only covers basic needs and does not provide prophylaxis. These women are usually immunised at the birth of the first child. If the second child is also rhesus positive, the fetal red cells enter the mother's circulation from 6-7 weeks. These cells express the D antigen quite strongly and lead to a booster of the "dormant" anti-D from the first pregnancy in the mother.
Such pregnancies must under no circumstances be cared for in a peripheral hospital. A pregnant woman with active immunisation should immediately be referred to a high-risk outpatient clinic of a specialised hospital. It does not always have to be anti-D either, but as soon as it has been established that the antibody found can cause Morbus Haemolyticus Fetalis (MHF), she must be referred. For most of the "dangerous" antibodies, which are boosted by antigens of the fetal erythrocytes, the titer also increases during pregnancy. Anti-D, in particular, can reach almost unbelievable levels. The titer is measured by agglutination of heterozygous D-positive cells with maternal serum. The serum is always diluted 1:1 in a geometric sequence (dilution factor: 1,2,4,8,16,32,64,128 etc.) This dilution, which still results in an agglutination level of 1, is given as the titer level. Most laboratories round from about 1000, so 2000, 4000 etc. The highest titer I have seen was a titer of 120000. The mother's serum was thus diluted 1:120,000 (!!!) and still had an anti-D concentration that could agglutinate Rhesus-positive cells. The child was severely ill (MHF), but with the help of intrauterine transfusions, it was born almost on term and healthy except for a slight anaemia with jaundice. Afterwards, it did not need an exchange transfusion, but only a few extra hours under the UV light.
However, one should not rely on the titer level. Antibodies of the Kell system can do a lot of damage even though the titer is only 4 or 8.
Some antibodies were already present before the pregnancy and occur naturally. For such women, the specialist in the special clinic will decide the treatment to be given. And not every woman who has an antibody during pregnancy needs treatment either. But this decision must come from someone who deals with it daily and not just has book knowledge.
The treatment of foetuses suffering from fetal haemolytic disease requires a great deal of experience in terms of knowledge, diagnostics and treatment techniques. With good care, there is only a low risk for the child, whereas poor care is associated with a high risk of stillbirth.
D-VARIANTs
The D antigen is the largest protein of the erythrocytic cell membrane, which it crosses with 12 loops. Due to its structure, it is assumed to be a transporter - however, the actual function of D has not been definitively clarified.
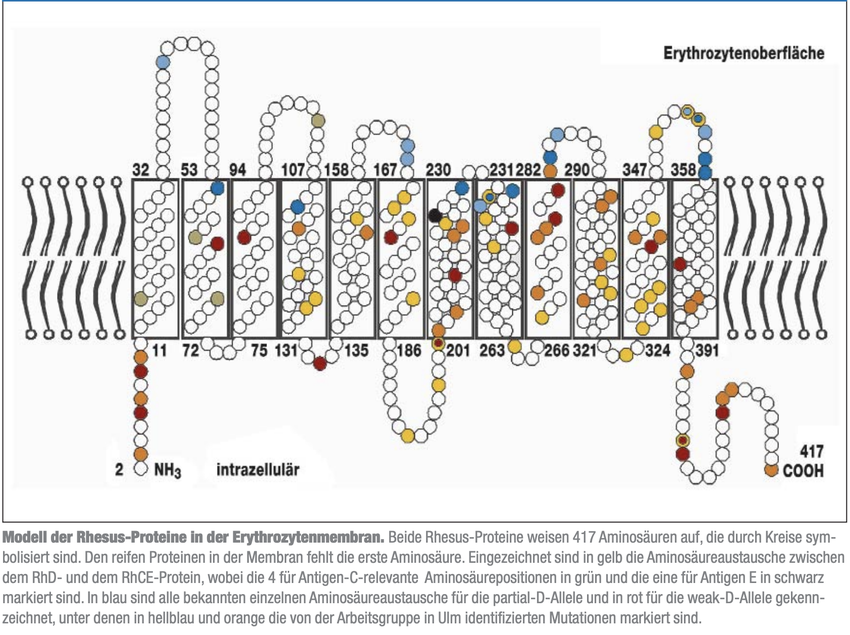
This illustration is the most famous illustration of the rhesus D antigen. Each coloured bead shows a mutation. There is a hypothesis that mutations within the membrane do not cause external changes. Thus no antibody formation is triggered in the carrier by the regular D antigen (in the case of a transfusion or pregnancy).
However, it is a hypothesis that is not easy to prove. Due to the regulation that all D-variant carriers need to be transfused with Rhesus D negative blood, it is very uncommon for them to be transfused with Rhesus D positive blood after all. And even in the case of Rhesus D-negative persons, immunisation with antibody formation does not always occur.
One only needs at least one case in which antibody formation has been proven for a particular variant to confirm the possibility of immunisation. Conversely, it is much more difficult because a lack of immunisation in one patient does not exclude it in another. Only for the most common variants, such as D weak type 1, 2 and 3, has sufficient data been collected to confirm no antibody formation in their carriers.
Full paper (in German):
Genetik der Rhesus-Blutgruppensystems von Prof. Willy Flegel, Deutschen Ärzteblatt 10/2007
D protein shows high polymorphism, resulting in attenuated or altered RhD antigens. In the past, the variants were classified as D weak or partial D. D weak meant reduced expression of the normal antigen on the erythrocytes. It was assumed that a normal D antigen could not immunise patients with weak D. Conversely, partial D was only partially expressed antigen - parts could be missing or altered. If a patient with a partial D was transfused with rhesus-positive, he could develop alloantibodies against the standard protein.
However, it soon became apparent that this classification was too simple. The D weak variants can also have changes that result in weakened expression. There are now countless D variants and new ones are constantly added. They have been collected for years on the Rhesus-Site of the University of Ulm.
TRANSFUSION RECOMMENDATION FOR D-VARIANTS
Only a few D variants are known with sufficient certainty that the carriers cannot be immunised by regular D. These are D weak types 1, 2 and 3. Patients with these D variants can be transfused with RhD POSITIVE blood and the women do NOT need Rh prophylaxis during pregnancy.
All other D variants should be transfused RhD NEGATIVE, and pregnant women with a D variant NEED Rh prophylaxis.
In addition to the common antigens in the rhesus system (C, D, E, c, e, Cw), there are over 50 other antigens, but these rarely cause the formation of an alloantibody.
REFERENCES:
1. Rhesus Site. Franz F. Wagner, Willy A. Flegel. Transfus Med Hemother 2014;41:357–363
2. Genetik der Rhesus-Blutgruppensystems von Prof. Willy A. Flegel, Deutschen Ärzteblatt 10/2007
Last update on 03.08.2023.
